The Disease
Systemic lupus erythematosus (SLE) is a multifactorial chronic autoimmune disease, marked by the presence of autoantibodies to nuclear antigens. It is characterized by circulating immune complexes that deposit in tissues, leading to multiorgan damage, with kidneys and skin primarily affected.
Interleukin 23
Interleukin-23 (p19 and p40 subunits) is a crucial cytokine implicated in chronic inflammation and autoimmunity, associated with various diseases like psoriasis, psoriatic arthritis, and systemic lupus erythematosus (SLE). IL23 has emerged as a promising therapeutic target for chronicinflammatory and autoimmune diseases.
Our preclinical testing tools
Biomedcode has recently added to its collection of unique animal models of human disease, the human IL23p19 (TghIL23p19) and the human IL12p40 (TgK14hIL12p40) mouse model, spontaneously developing lupus-like autoimmune pathologies due to the replacement of the gene’s 3’UTR with that of beta-globin that is not subject to post-transcriptional regulation. Expression pattern is determined by the promoters used, that is the gene’s own promoter for TghIL23p19 and the human keratin 14 promoter for TghIl12p40.
Read-Out Parameters
Validated platform
TghIL23p19-driven lupus-like disease
Mice overexpressing human IL23p19 subunit under its own human promoter, develop lupus-like disease. Starting treatment at the age of 10 weeks, disease is ameliorated after anti-human IL23p19 (guselkumab).
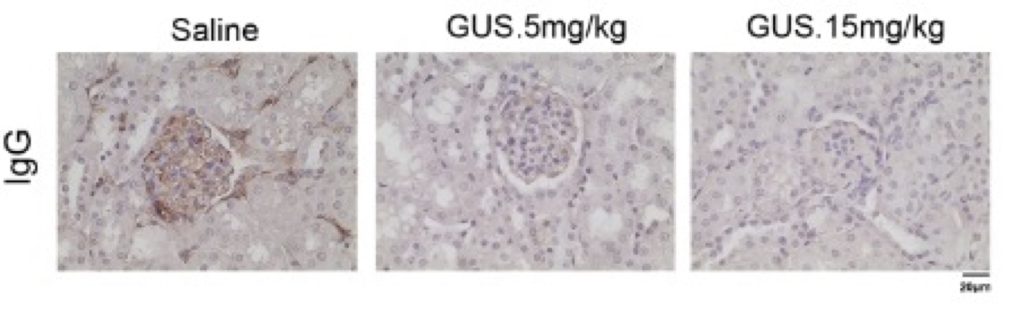
IgG deposits in the TghIL23p19 kidney. Treatment with guselkumab ameliorates the disease symptoms.
Competitive Advantage
TghIL23p19 model efficiently recapitulates human lupus pathology and in combination to standardized evaluation procedures it offers the opportunity for reliable evaluation of the efficacy of anti-human IL23p19 biologics and biosimilars as well as novel lupus treatments.