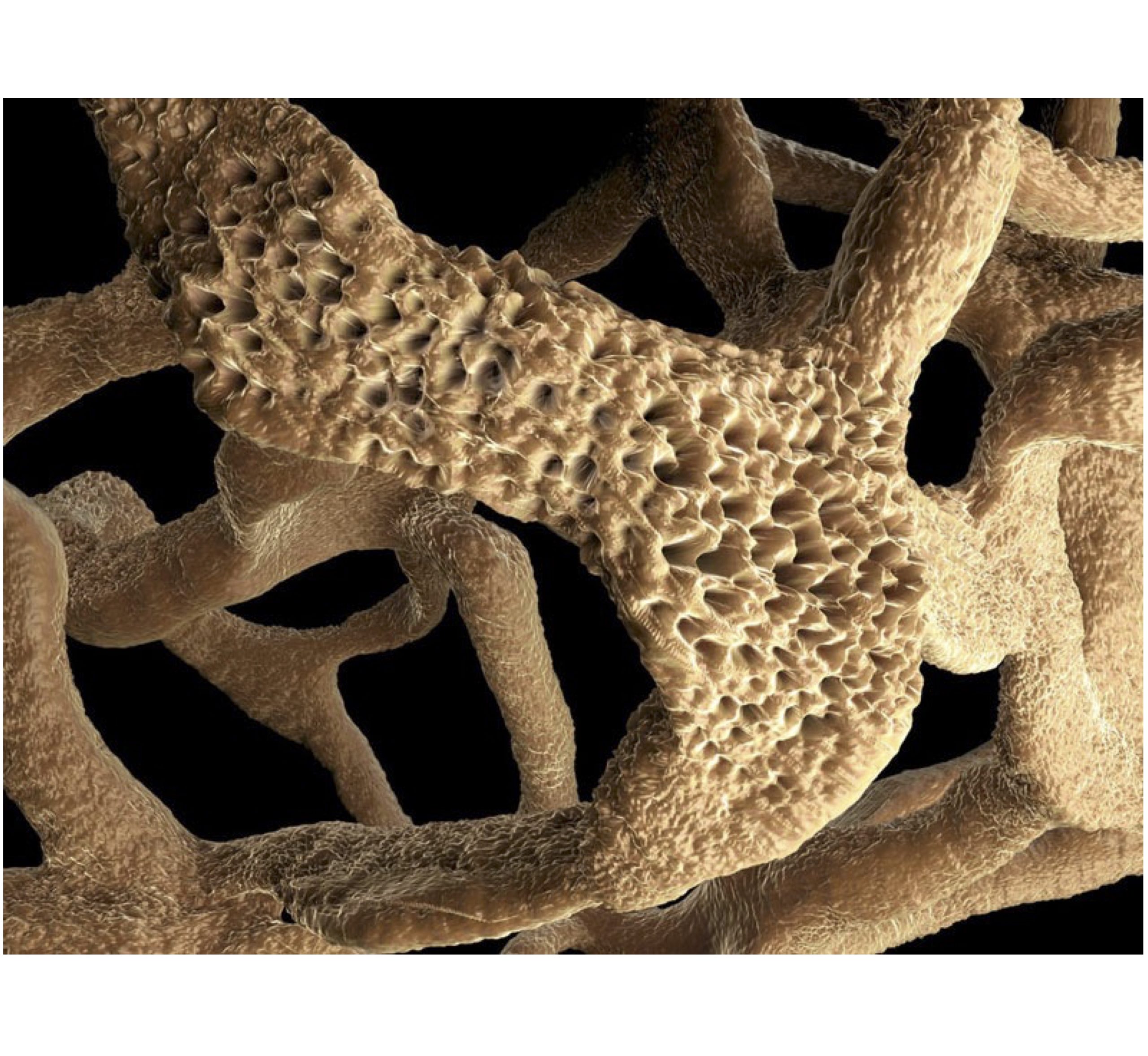


Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease characterized by irreversible fibrosis. It involves the transition of fibroblasts to myofibroblasts, the tissue stiffening and alveolar epithelium injury eventualy causing irreversible and progressive lung damage.
Various methods have been employed to simulate pulmonary fibrosis for the purpose of evaluating potential anti-fibrotic therapeutic treatments. Apart from the use of IPF animal models where the pathology is induced through the endotracheal administration of bleomycin and the development of 2D cell cultures for the study of fibrosis mechanisms, three-dimensional (3D) cultures have also emerged as valuable tools for modeling IPF.
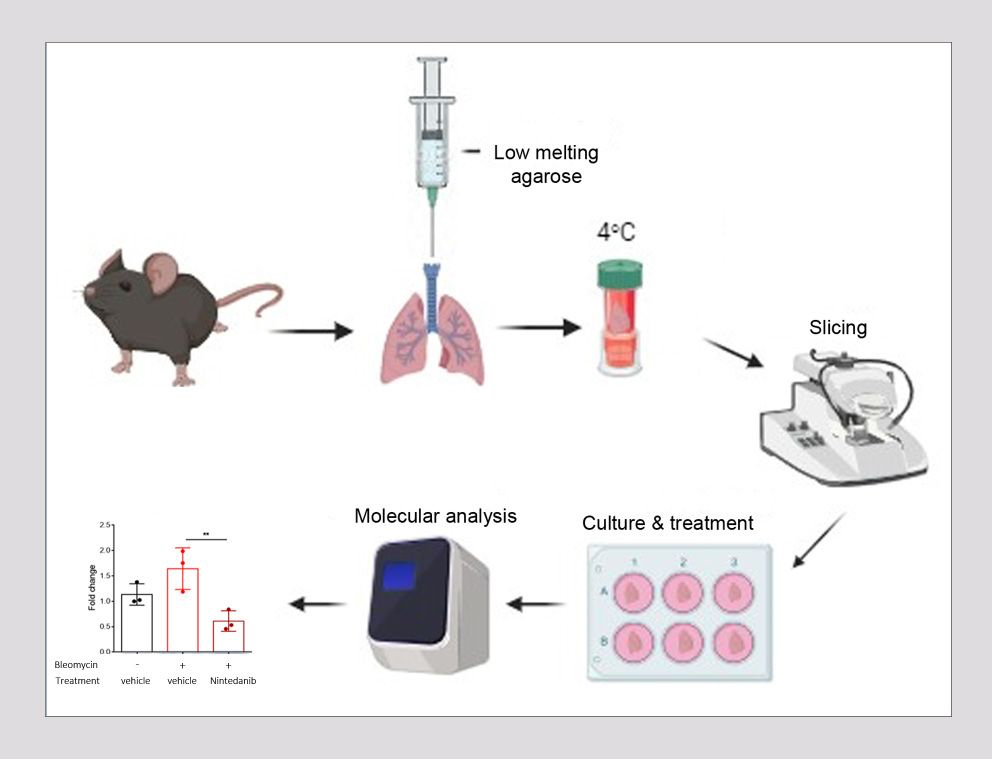
One such 3D culture approach is the generation of precision-cut lung slices (PCLS), where thin slices with precise thickness are obtained from fresh tissue with the use of a vibrating microtome. These slices recapitulate the in vivo lung environment by offering a preserved tissue structure with intact cell-cell and cell-extracellular matrix interactions, allowing for the study of molecular mechanisms between different lung cell populations and facilitating lung degeneration and alveologenesis. The advantage of this approach is that it allows for the generation of multiple slices from the lungs of a single mouse , thereby greatly reducing the number of animals required for individual experiments.
As it has been shown that PCLS obtained from IPF patients or bleomycin-induced mouse lungs, exhibit reduced expression of fibrotic markers following TGF-β inhibition, Biomedcode is currently developing a preclinical ex vivo tool using PCLS derived from bleomycin-induced mouse lungs to screen ex vivo the therapeutic efficacy of anti-fibrotic drugs. Using Nintedanib, an intracellular tyrosine kinase inhibitor that targets fibroblast recruitment, proliferation and differentiation, as a positive control and through the assessment of fibrosis biomarkers such as fibronectin, alpha Sma and collagen 1A1, we have generated experimental evidence supporting that PCLS from IPF-affected mouse lungs can nicely serve as a powerful preclinical tool to assess the anti-fibrotic effect of test articles targeting inflammation and fibrosis.
Graph created by bioRender
TNF is a pleiotropic cytokine serving important functions in pathophysiology, as it plays regulatory role in the growth, differentiation and death of immune and non-immune cells. Its functions are mediated through binding to two transmembrane receptors, TNFR1 and TNFR2, that deliver signals to activate a variety of responses ranging from proliferation to apoptosis and necrosis.
Dysregulation of TNF has been implicated in immune mediated inflammatory diseases including rheumatoid arthritis, inflammatory bowel disease, psoriasis, scleroderma, systemic lupus erythematosus, atherosclerosis as well as autoimmune pathologies, cancer etc.
Since the approval of the first TNF inhibitors, back in 1998, TNF blockade has been used as a successful approach in treating a broad range of pathologies, having nonetheless the expected compromises stemming from the severe side effects that result from the blockade of the whole range of the physiological functions of TNF, with the increased susceptibility to infections being one of the majordrawbacks of this therapeutic approach.
The pioneering work of two Greek scientists, Kassiotis and Kollias, published in 2001 in JEM (193:427) and JCI (107:1507), shed light for the first time on the importance ofuncoupling the TNFR1- and TNFR2-TNF signaling pathways. This work proposed that specific blockade of TNFR1 signaling could offer advantageous novel therapeutic approaches as they would suppress the TNF pro-inflammatory activity, leaving unaffected its TNFR2-dependent immunosuppressive activity. In the following years, indeed TNFR1 emerged as an attractive target towards ameliorating the detrimental effects of the TNF signalling pathway and various TNFR1 inhibitors are currently under development or in clinical trials.
A unique tool for testing anti-hTNFR1 therapeutics is the human TNFR1 knock in (hTNFR1KI) mouse model, that expresses human TNFR1 in the absence of its mouse counterpart. The hTNFR1KI mouse combined, as shown in the table below, with induced or spontaneous disease models offers a fully equipped armory for the evaluation of human therapeutics targeting TNFR1.
Preclinical Platforms for the evaluation therapeutics targeting human TNFR1
| Model | Duration | Read outs |
| Experimental Autoimmune Encephalomyelitis
MOG-induced EAE in hTNFR1KI mice |
25-30 days | -Weight
-Clinical Scoring -Histology |
| Spontaneous Arthritis
Tg197hTNFR1KI TNFΔARE/+ hTNFR1KI |
12 weeks
4-8 weeks |
-Weight
-Clinical Scoring -Histology |
| Induced Arthritis
Collagen Antibody Induced Arthritis (CAIA) in hTNFR1KI mice |
2 weeks | -Weight
-Clinical Scoring -Histology -Serum Cytokine levels |
| Acute Inflammation
TNF/GaIN induced inflammation in hTNFR1KI mice |
24 hours | -Serum cytokine levels-Survival |
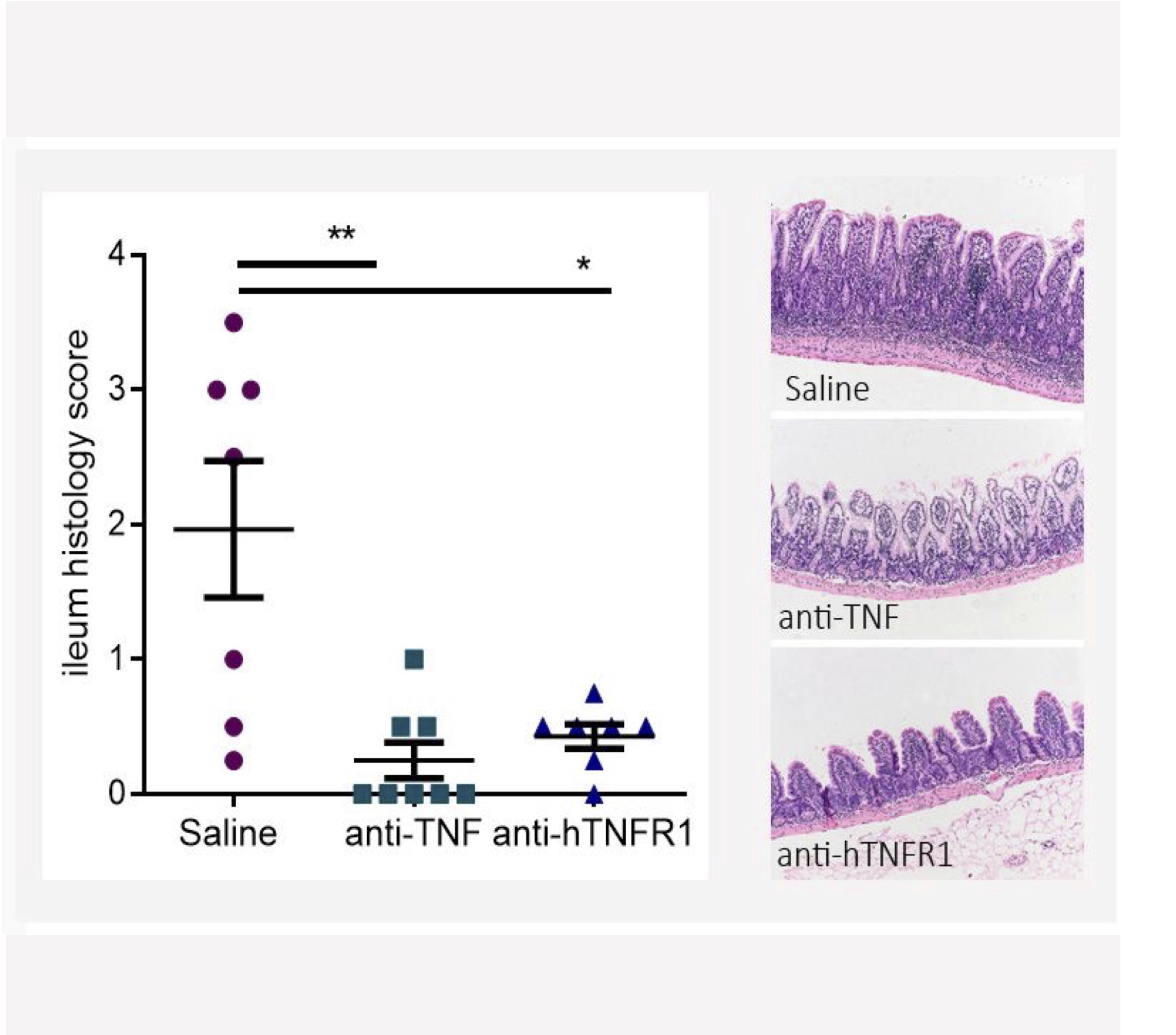
One of the models described above that has a particular interest due to its complexity and similarity to the human condition is the TNFΔAREhTNFR1KI mouse model that due to the dysregulated expression of TNF develops spontaneously intestinal inflammation, arthritis and cardiovascular disease, perfectly recapitulating many of the features that constitute the complexity of the human disease. TNFΔARE was the first mouse model that unequivocally established the causal role of TNF in the inflammatory response of Crohn’s-like ileitis, while further studies revealed numerous promising therapeutic targets some of which were later developed into approved drugs, such as Ustekinumab, vedolizumab, and natalizumab.
The TNFΔAREhTNFR1KI mouse model, faithfully reproduces all TNFΔARE phenotypes and is an ideal tool for translational research, supporting the drug discovery and development of biologics and small molecules targeting TNF, hTNFR1 as well as other potential therapeutic targets of Crohn’s-like ileitis.
Fibroblasts are responsible for creating and maintaining the extracellular matrix that normally supports all the connective tissues. Tumors have the ability to recruit fibroblasts (Cancer Associated Fibroblasts (CAFs)) that develop a fibrotic microenvironment that supports their growth while protecting them from the immune system. This fibroblast-generated scar tissue is associated with the reduced efficiency of chemotherapies and poor outcome of treatment.
A new publication in Nature Cancer provides evidence that reducing scar tissue with the PXS-5505 small molecule inhibitor can help to treat pancreatic cancer by making it more accessible to chemotherapy treatment. PXS-5505 is an inhibitor of the lysyl oxidase family of enzymes which causes collagen to build up around tumors creating fibrotic scar tissue that acts as a barrier inhibiting the access of chemotherapy agents. The study offers exciting evidence that therapeutic strategies that involve the targeting of tumor-associated stroma can offer several benefits to the treatment of cancer including improved chemotherapy responses, reduced metastatic burden and more importantly prolonged survival.
Read the full article in Nature Cancer

In response to the current outbreak of coronavirus disease (COVID-19), Biomedcode has implemented measures to help ensure the health and safety of our employees as well as their families but to also ensure the welfare of the mice under our care as well as the uninterrupted provision of services under the same high standards of quality.
Greece is currently implementing a “Stay At Home” order, intended to minimize the spread of the disease. In this context Biomedcode has adjusted its operation so that personnel that can work from home are doing so, while personnel essential for the proper operation of our animal facilities as well as the execution of contracted research activities, are provided with the necessary documentation to ensure their on-site presence.
With a long-standing mission to support biomedical research and drug development, Biomedcode is closely monitoring the scientific findings of this pandemic and is open to contributing with its preclinical mouse models in the global effort to fight this pandemic.
We will continue to take all necessary precautions and we will be happy to address any questions or concerns you may at info@biomedcode.com.

A recent article published in Science translational Medicine by Spector et al. set out to determine the contribution of fundamental research to the generation of new medicines. By tracking the scientific origins of some of today’s most “transforming” FDA-approved drugs, including medicines such as TNF blockers, ACE inhibitors, kinase inhibitors and others, they find that approximately 80% of the 28 medicines studied, originated as basic research discoveries by scientist seeking to understand a biological process or disease. It is noteworthy, that the path to drug discovery that starts with basic scientific research more often than not includes fundamental research work performed in mouse models of disease that provide invaluable insights and proof of concept on in vivo disease mechanisms and drug activity. One such example of the invaluable contribution of animal disease models in the path to drug discovery, is the human TNF transgenic Tg197 arthritis mouse model that in 1991 provided the first in vivoevidence that deregulated TNF production was causal to chronic polyarthritis (Keffer et al.).
