

Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease characterized by irreversible fibrosis. It involves the transition of fibroblasts to myofibroblasts, the tissue stiffening and alveolar epithelium injury eventualy causing irreversible and progressive lung damage.
Various methods have been employed to simulate pulmonary fibrosis for the purpose of evaluating potential anti-fibrotic therapeutic treatments. Apart from the use of IPF animal models where the pathology is induced through the endotracheal administration of bleomycin and the development of 2D cell cultures for the study of fibrosis mechanisms, three-dimensional (3D) cultures have also emerged as valuable tools for modeling IPF.
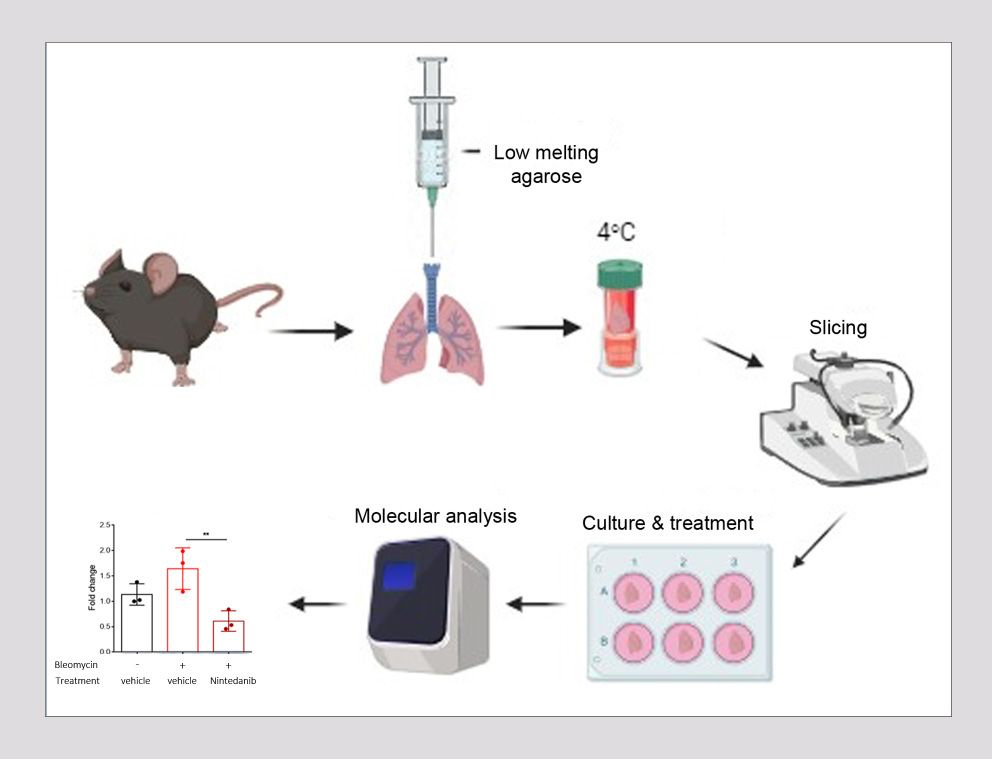
One such 3D culture approach is the generation of precision-cut lung slices (PCLS), where thin slices with precise thickness are obtained from fresh tissue with the use of a vibrating microtome. These slices recapitulate the in vivo lung environment by offering a preserved tissue structure with intact cell-cell and cell-extracellular matrix interactions, allowing for the study of molecular mechanisms between different lung cell populations and facilitating lung degeneration and alveologenesis. The advantage of this approach is that it allows for the generation of multiple slices from the lungs of a single mouse , thereby greatly reducing the number of animals required for individual experiments.
As it has been shown that PCLS obtained from IPF patients or bleomycin-induced mouse lungs, exhibit reduced expression of fibrotic markers following TGF-β inhibition, Biomedcode is currently developing a preclinical ex vivo tool using PCLS derived from bleomycin-induced mouse lungs to screen ex vivo the therapeutic efficacy of anti-fibrotic drugs. Using Nintedanib, an intracellular tyrosine kinase inhibitor that targets fibroblast recruitment, proliferation and differentiation, as a positive control and through the assessment of fibrosis biomarkers such as fibronectin, alpha Sma and collagen 1A1, we have generated experimental evidence supporting that PCLS from IPF-affected mouse lungs can nicely serve as a powerful preclinical tool to assess the anti-fibrotic effect of test articles targeting inflammation and fibrosis.
Graph created by bioRender
