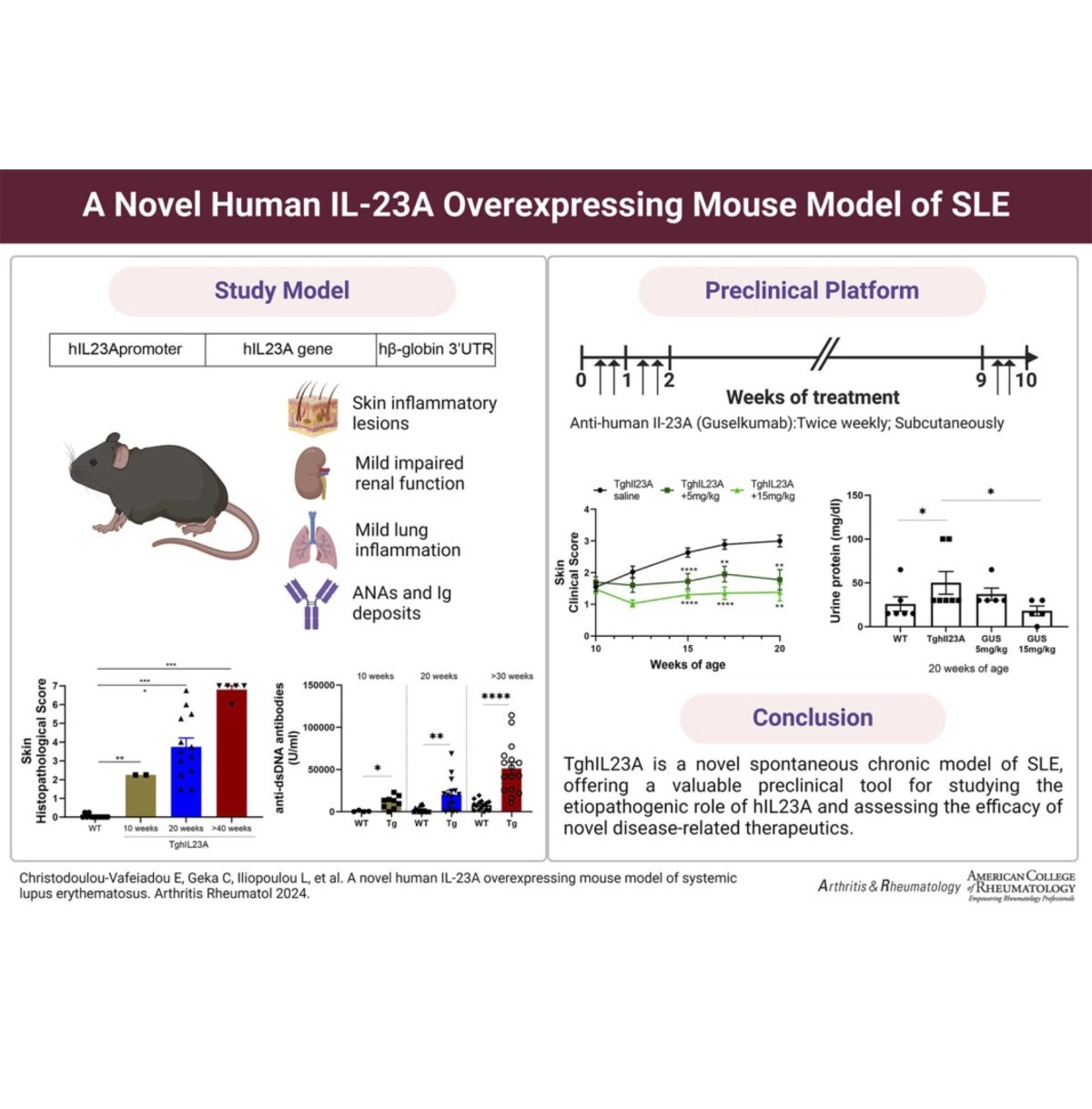
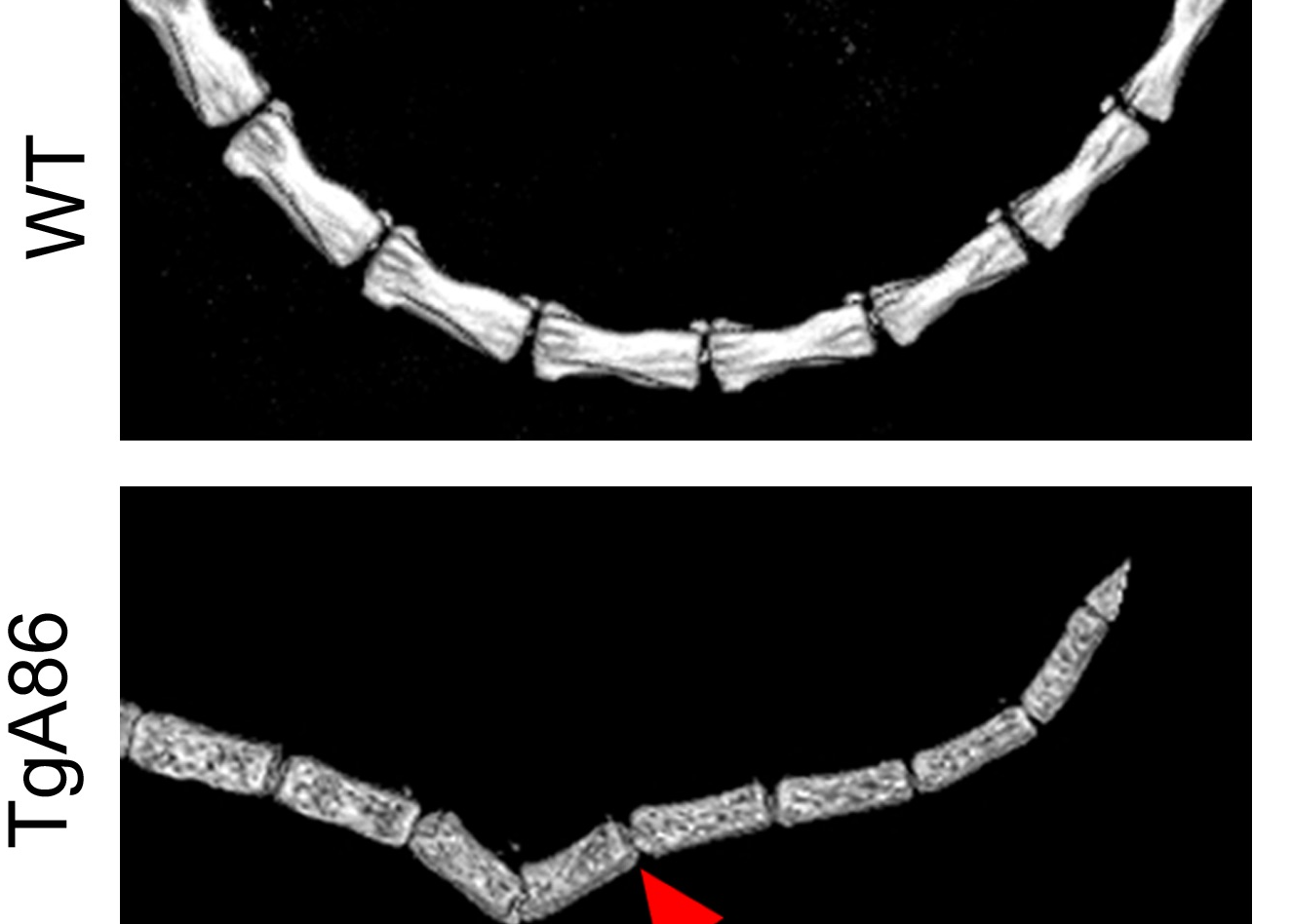
This newest addition in our collection of humanized mouse disease models integrates characteristics of the human Systemic Lupus Erythematosus complexity and develops a chronic multiorgan autoimmune disease marked by proteinuria, anti-dsDNA antibodies, severe inflammatory lesions in the skin and milder pathologies in the kidneys and lungs.
This novel model of lupus can prove to be an invaluable translational tool for studying the aetiopathogenic role of the IL23 cytokine in SLE and for use as a preclinical tool to assess the efficacy of novel lupus therapeutics.
Published in Arthritis Rheumatol. 2024 Feb 15. doi: 10.1002/art.42830.