
The Disease
Psoriasis is a frequently occurring inflammatory skin disorder characterized by hyper proliferating keratinocytes and massive infiltration of leukocytes. It affects about 25 million people in North America and Europe and is likely the most prevalent immune-mediated skin disease in adults. Psoriasis treatment needs long term drug application, generating a high volume of sales that is predicted to further increase as the demand for efficacious treatment of psoriasis in emerging countries is rising.
Our preclinical testing tools
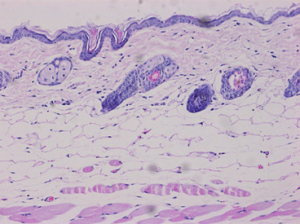
The topical application of IMQ on the shaved back skin of the mouse induces a psoriasis-like skin condition exhibiting most of the human psoriasis pathology characteristic features including acanthosis, parakeratosis, infiltration of immune cells and involvement of the IL23/ IL17/ IL22 pathway.
Psoriasis Preclinical Evaluation Platforms
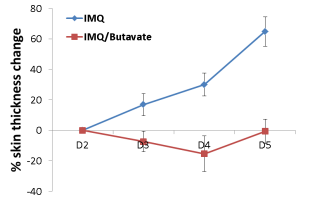
IMQ is applied on the shaved back of mice daily for 5 consecutive days. Signs of psoriasis appear from day 2 onwards and involve skin erythema, scaling and thickening. The severity of the pathological signs is assessed daily and treatment with corticosteroids and anti-hIL17 reverses symptoms.
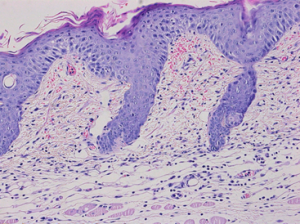
Read-Out Parameters
Daily body weight measurements reflecting the general health status
In vivo scoring of skin erythema, scaling and thickness reflecting the progress of the pathology
Spleen weight to body weight ratio (for Wild-Type animals only) reflecting the disease related immune activation
Histopathological scoring
Validated platforms
IMQ-induced psoriasis in Wild-Type mice
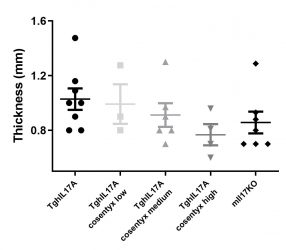
IMQ-induced psoriasis in TghIL17 mice
Biomedcode’s unique IL17 humanized mice express exclusively human IL17. TghIL17 mice have normal phenotype and in combination with IMQ induction protocols offer a unique tool for the evaluation of the efficacy of anti-human IL17 therapeutics in the treatment of psoriasis.
Competitive Advantage
IMQ induced psoriasis reproduces the majority of the pathology features of the human condition. Standardization and validation of the model by our experienced scientific personnel provides a reliable preclinical evaluation platform for the efficacy assessment of candidate psoriasis therapeutics, while induced psoriasis on humanized IL17 mice (TghIL17) allows the evaluation of biologics targeting human IL17.