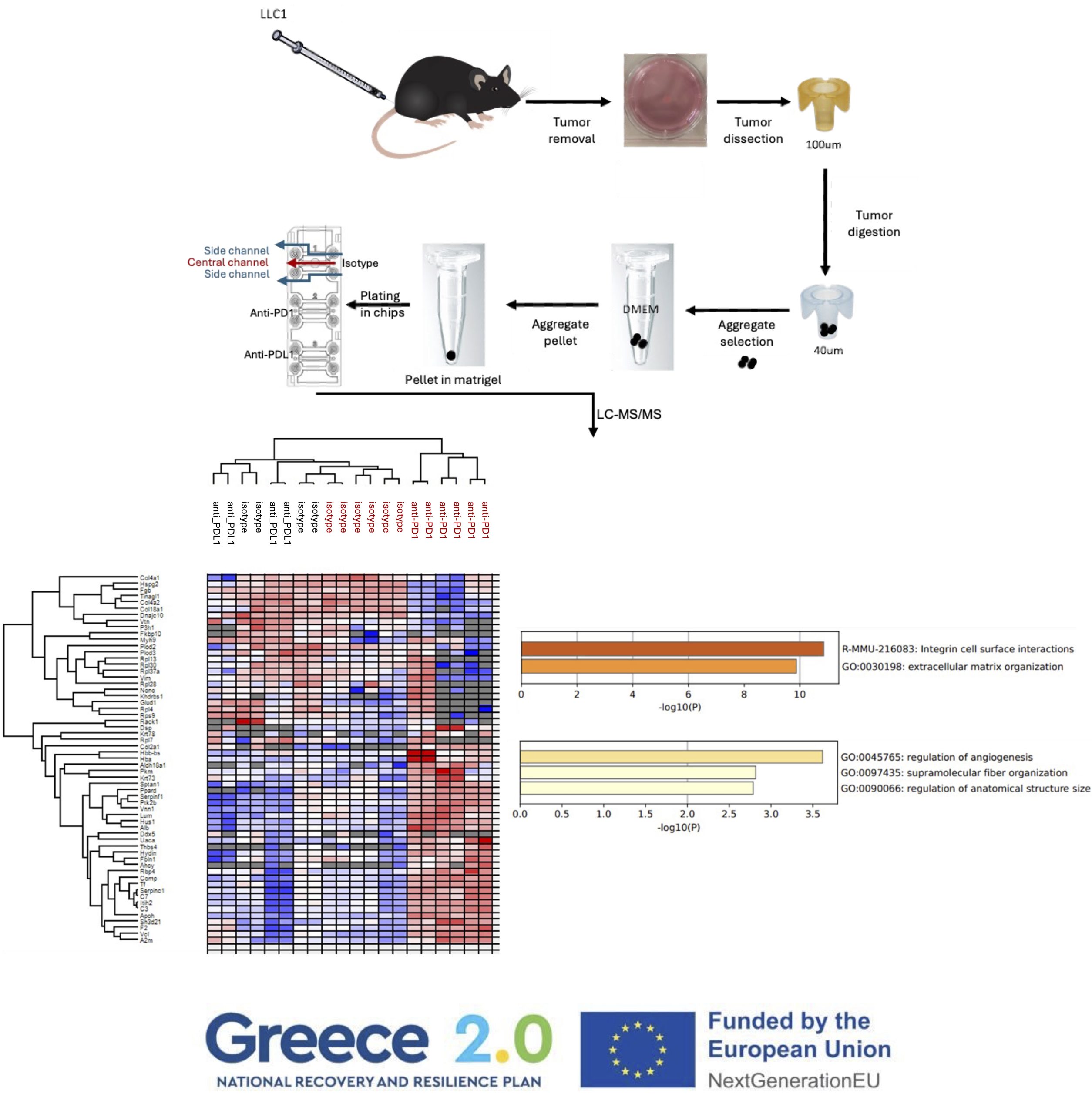
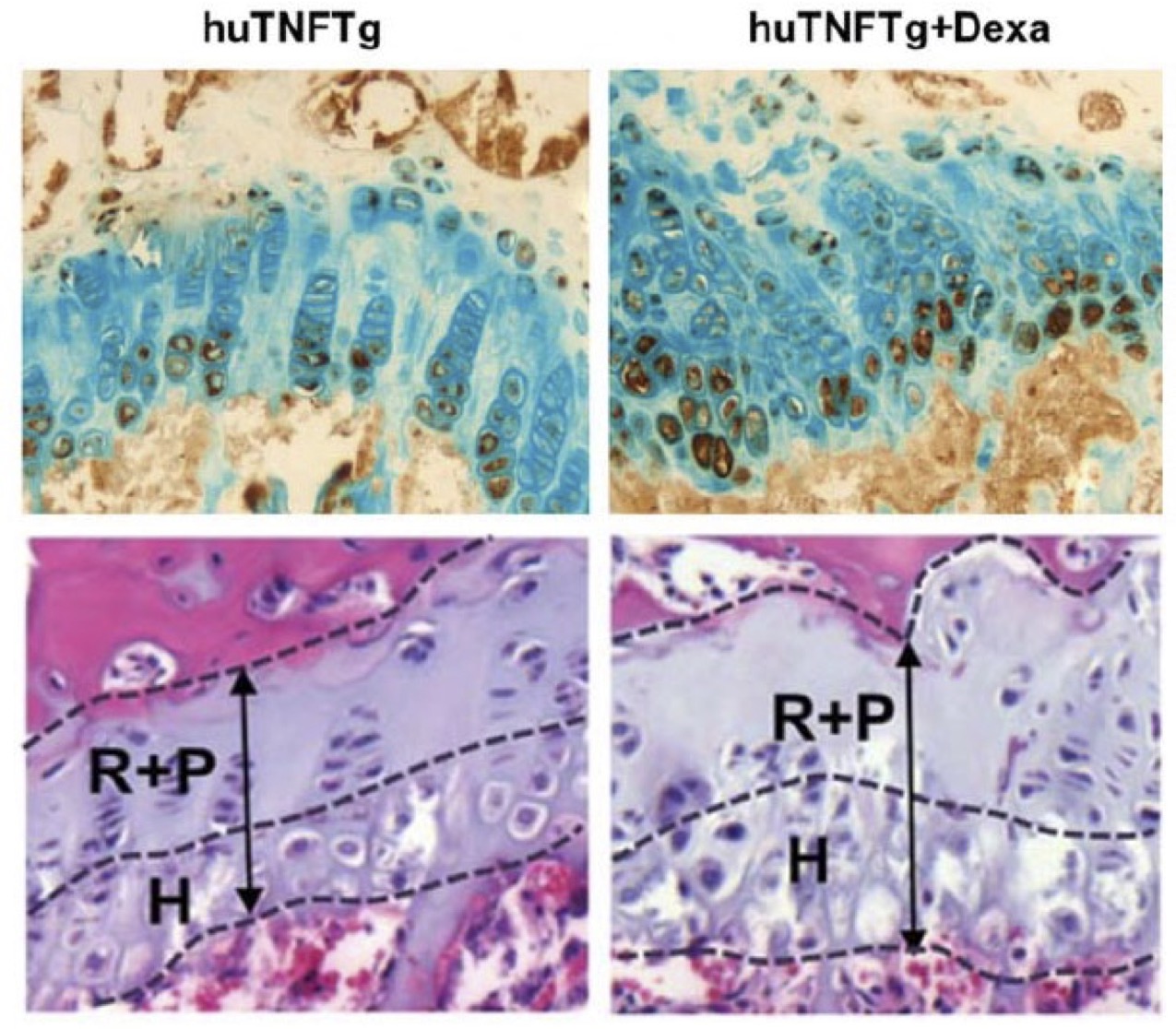
Walking on the side of researchers striving to establish an assay for the prediction of the response of human lung cancer patients to cancer immunotherapy, we have established a 3D microfluidic tumour microculture system that involves the seeding of LLC (Lewis Lung Cancer) syngeneic mouse tumour aggregates in the central hydrogel channel of an Aim Microfluidic Chip and the injection of anti-PD1 or anti-PDL1 antibodies in the two flanking side channels.
Proteomics analysis proteomics of the untreated vs anti-PD1 treated tumour samples revealed the signature of the response to treatment (A). Metascape analysis of increased (B) or decreased ( C) protein expression after anti-PD1 treatment revealed the biological pathways involved.
With this data we have the proof of concept to support that a similar humanized PDL1/PD1 system can be used for screening the responses of human patients to anti-human PD1 therapeutics.
This work was performed under the European funded program “Next Generation EU” Greece 2.0, https://greece20.gov.gr/en/) BioOnChip, “Development of a Bronchoscopic Biopsies-On-Chip platform for immunotherapy drug screening in non-small cell lung cancer” that aimed to explore the applicability of an innovative 3D microfluidic microculture in predicting real-time responses to PD1-blockade in NSCLC patients, that, if efficient, might have important socioeconomic impact.


INFRAFRONTIER has achieved two major milestones in the first half of 2024, with two collaborative projects being funded by the European Commission INFRAPLUS and PRIMTECH3R
Biomedcode participates in both projects together with other partners and collaborators:
Helmholtz Munich – ERA-LEARN – Institut Clinique De La Souris ICS – CIPHE – Centre d’Immunophénomique – University of Oulu – Karolinska Institutet – CSIC – Centro Nacional de Biotecnología (CNB-CSIC) – Consiglio Nazionale delle Ricerche – Vetmeduni – BSRC Alexander Fleming – Universitat Autònoma de Barcelona – UK Research and Innovation – Mary Lyon Centre at MRC Harwell – MRC National Mouse Genetics Network – Biomedcode – AKITA, by Finnadvance – CNRS – Calouste Gulbenkian Foundation Instituto Gulbenkian de Ciencia – The Netherlands Cancer Institute – Antoni van Leeuwenhoek – The Hospital for Sick Children – Institute of Molecular Genetics of the Czech Academy of Sciences – Czech Centre for Phenogenomics – UMCG research – EMBL – INSERM and Aarhus University