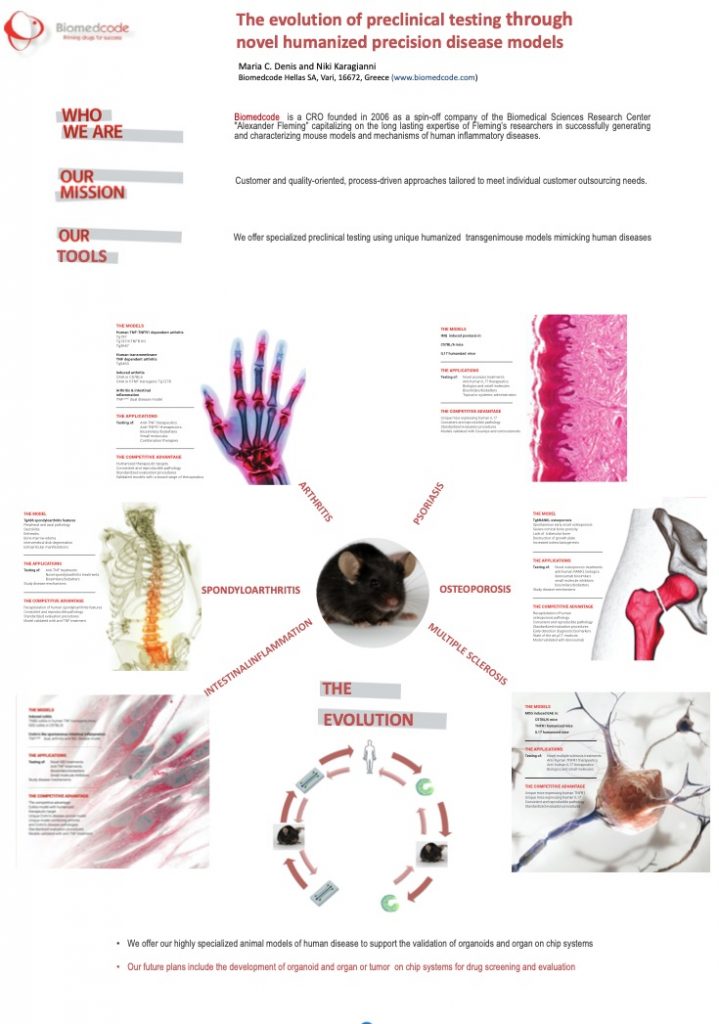
The evolution of preclinical testing through novel humanized precision disease models
Background: In search for advanced therapies for human disease, the translational value of preclinical work is unquestionable. There is however a pressing need to develop animal models that closely reflect the pathogenic mechanisms as well as the complexity of the human disease.
Aim and Methods: Biomedcode develops advanced mouse models with high predictive power for the therapeutic efficacy of human therapeutics. These models are humanized for key pathogenic players such as TNF, TNFR1, IL17, RANKL, IL23p19 and others and, either spontaneously or following induction, develop chronic inflammatory conditions such as arthritis, IBD, osteoporosis, spondyloarthritis, psoriasis etc. Biomedcode’s arthritis mouse models have provided the in vivo proof-of-concept and supported the successful IND filing of several novel and biosimilar human therapeutics, including Infliximab (Remicade®) whose anti-TNF therapeutic activity was first validated in the human TNF transgenic arthritis mouse models.
Conclusion: Using standardized procedures and high end in vivo and ex vivo read outs, Biomedcode maximizes the scientific output from each experimental model closely following the 3Rs and maximizing the translational value of the models. As we now move to the era of advanced therapies, it is critical to use optimized mouse models that guarantee similarity to human disease and allow the accurate transition from bench to bedside and back. Optimized mouse models will also allow the validation of preclinical testing approaches based on organoid or microphysiological systems, supporting thus combined preclinical testing of advanced therapies and application of the Replacement principle.