Biomedcode has been recognized by the Pharma Tech Outlook magazine as one of the top 10 CROs of the year 2020. An interview with Biomedcode’s CEO and CSO is published in this month’s special edition on Europe’s CROs.


Biomedcode has been recognized by the Pharma Tech Outlook magazine as one of the top 10 CROs of the year 2020. An interview with Biomedcode’s CEO and CSO is published in this month’s special edition on Europe’s CROs.

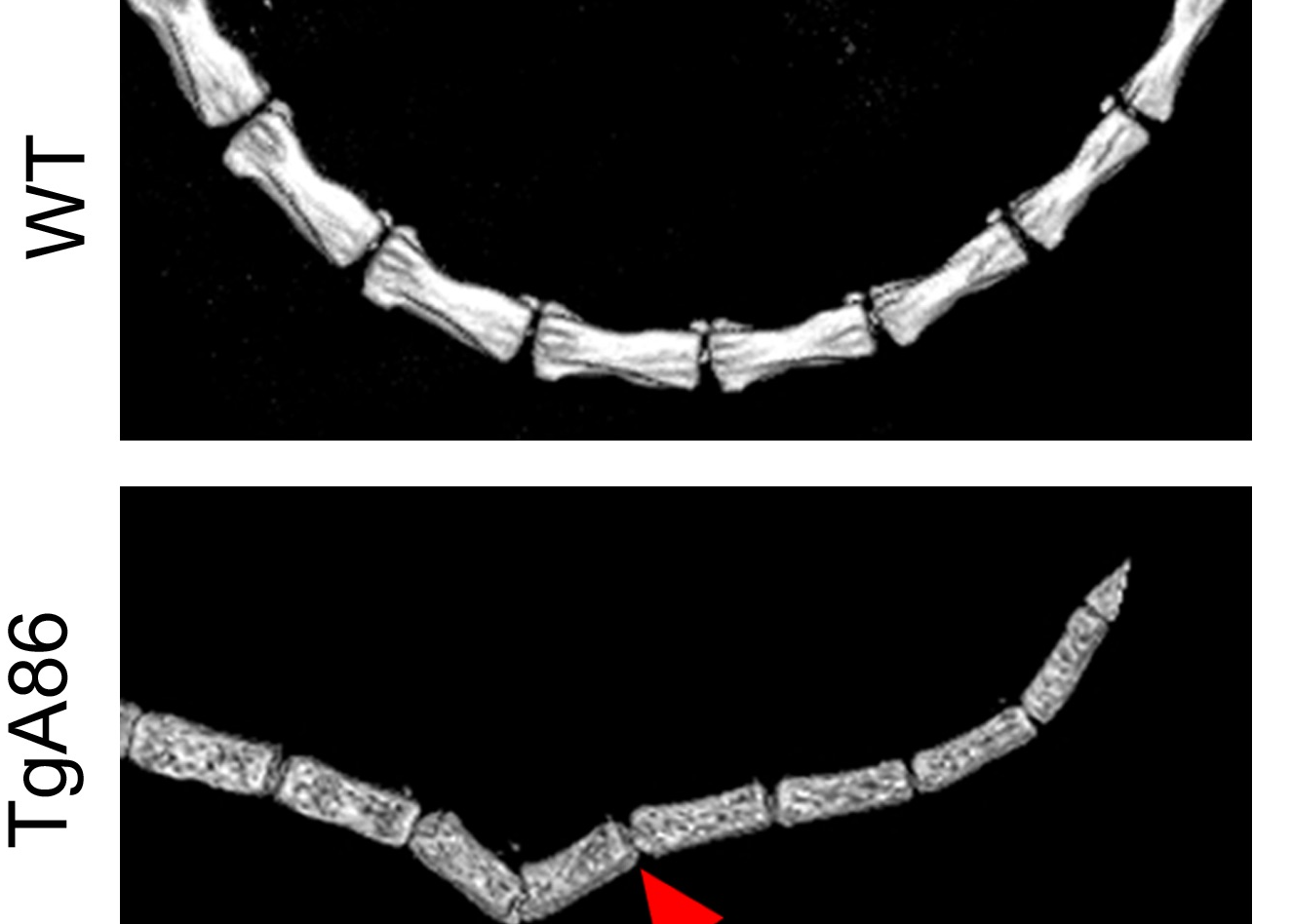
With a new publication in Arthritis Research and Therapy, entitled “Ectopic bone formation and systemic bone loss in a transmembrane TNF-driven model of human spondyloarthritis”, Biomedcode in collaboration with George Kollias Lab at BSRC Al. Fleming, introduce the TgA86 transmembrane TNF transgenic mouse as a novel model of human spondyloarthritis (SpA).
The authors show that the TgA86 mouse model develops spontaneously peripheral arthritis and axial pathologies that closely reproduce key pathogenic features of human SpA, including distinct stages of inflammation and ectopic new bone formation. This is a chronic and complex disease model that similar to human patients also develops extraarticular comorbidities such as heart valve pathology and systemic bone loss. As with human patients in the clinic, all the pathologies of the TgA86 mouse model are reversed following early treatment with anti-hTNF therapeutics.
This novel model of SpA that captures not only specific features, but also the complexity of human disease, can prove to be an invaluable translational tool in the study of SpA pathogenesis as well as in the evaluation of human therapeutics.
Published in Arthritis Research and Therapy 2020 Oct 6;22(1):232. doi: 10.1186/s13075-020-02327-4.

Biomedcode is excited to participate as one of the 63 European companies that have been selected to join the EUGATEWAY Healthcare and Medical Technologies Business Mission to Singapore on 8-11 December 2020. Due to the COVID-19 pandemic this will be a virtual event and we look forward to discussing with potential new partners and clients about future collaborations!
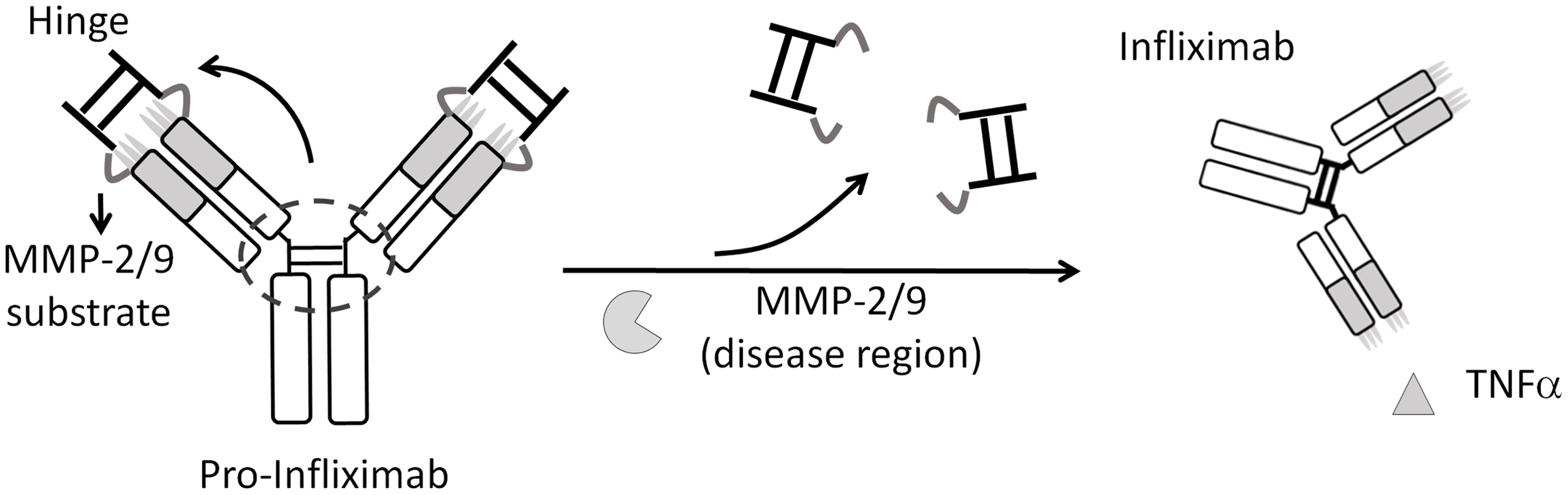
Pro-Infliximab provided equivalent therapeutic efficacy to Infliximab while maintaining mouse immunity against Listeria infection, leading to a significantly higher survival rate (71%) than that of the Infliximab treatment group (0%).
Published in PLoS Biology 2019 Jun 13;17(6):e3000286. doi: 10.1371/journal.pbio.3000286.

In response to the current outbreak of coronavirus disease (COVID-19), Biomedcode has implemented measures to help ensure the health and safety of our employees as well as their families but to also ensure the welfare of the mice under our care as well as the uninterrupted provision of services under the same high standards of quality.
Greece is currently implementing a “Stay At Home” order, intended to minimize the spread of the disease. In this context Biomedcode has adjusted its operation so that personnel that can work from home are doing so, while personnel essential for the proper operation of our animal facilities as well as the execution of contracted research activities, are provided with the necessary documentation to ensure their on-site presence.
With a long-standing mission to support biomedical research and drug development, Biomedcode is closely monitoring the scientific findings of this pandemic and is open to contributing with its preclinical mouse models in the global effort to fight this pandemic.
We will continue to take all necessary precautions and we will be happy to address any questions or concerns you may at info@biomedcode.com.

Biomedcode is pleased to announce that, the T2EDK program -Spondylitis Evaluation Platform via non invasive Imaging Application tools (SEPIA)- initiated in collaboration with the developer of novel breakthrough imaging systems company BIOEMTECH, has been financed by the European Union and Greek national funds through the operational program Competitiveness, Entrepreneurship and Innovation under the call RESEARCH-CREATE-INNOVATE. SEPIA will combine the efforts of two innovative companies aiming to develop precision imaging tools for the evaluation of the severity of Spondyloarthritis pathology developed in the TgA86 established mouse model of the pathology. Integration of these imaging tools in the sprondyloarthritis drug efficacy evaluation platform will support the preclinical development of novel therapies for Spondyloarthritis.

Tg3647 mice over-express human TNF that leads to the gradual development of spontaneous slow progressing chronic inflammatory polyarthritis with 100% penetrance.

Targeting TNF-α as a treatment modality has shown tremendous success, however there are several limitations associated with the current anti-TNF-α biologic drugs including: immunogenicity, life-threatening infections, resistance to treatment, complexity of manufacture and cost of treatment. Ubah et al. report the in vivo efficacy of novel anti-TNF-α formats generated from molecular engineering of variable new antigen receptors (VNARs), originally derived from the immune system of an immunized nurse shark.
Published in Frontiers in Immunology 2019 Mar 22;10:526. doi: 10.3389/fimmu.2019.00526.
Collagen antibody-induced arthritis (CAIA) is a simple mouse model of rheumatoid arthritis that can be used to address questions relating to the pathogenic mechanisms of the disease and serves as a platform for the evaluation of candidate therapeutic agents.
Biomedcode has standardized the model in
and developed platforms to allow the evaluation of different arthritis therapeutics.